Different Types of Dyes and Their Properties
In order to grasp the concept of dyeing, one needs to have a clear understanding of the different types of dyes that are currently available on the market. Before getting into that, let’s explore the most obvious question first–
What is Dye?
A dye is a coloring material that is used for imparting color to different substances or altering the color of something. Dyes have chromophores and auxochromes which are responsible for their color and substantivity.
Types of Dyes
Dyes can be classified based on various parameters. However, we’ll take a look at four most pronounces ones –
Based on the Source
- Natural dyes
- Synthetic dyes
Based on Ionic Nature
- Anionic dyes: Reactive dye, acid dye, etc.
- Cationic dyes: Basic dye.
- Non-ionic dyes: Disperse dye.
Based on Chromophoric Groups
- Azo dyes
- Anthraquinone dyes
- Nitro and nitroso dyes
- Triarylmethane dyes
- Indigo dyes
Based on Chemical Structure
Ready-made Dyes
- Water-soluble dyes: Reactive dye, direct dye, Acid dye, etc.
- Water-insoluble dyes: Vat dye, Sulphur dye, etc.
Ingrain Dyes
- Mineral colors
- Azoic colors
- Oxidation colors
Basic Theory of Dyeing
In dyeing, color is transferred to a textile material to make it permanently colored. In the textile industry, generally, it is done by different machines and consists of several steps.
Now coming to the basic dyeing theory, the interaction between dyes and the textile material along with different dyeing auxiliaries consists of 3 general stages.
- Dye migration from the solution to the surface of the fiber, which can also be called as adsorption.
- Dye diffusion to the interior of the fiber from the surface, which can also be called as absorption.
- Dye fixation by different types of bonds or entrapping inside the fiber pores.

Different types of methods are available for textile coloration.
- Direct dyeing
- Yarn dyeing
- Garment dyeing
- Stock dyeing
- Top dyeing
- Piece dyeing
- Dope dyeing
Different Dyes with Their Properties
Now, we will discuss different dyes with their primary dyeing mechanism.
Direct Dyes
We can apply these dyes directly on the fiber as they have a strong affinity and so they are called direct dyes. Mainly these dyes are sodium salts of sulphonic acid or carboxylic acid, and their leading chromophoric group is azo. They are also known as substantive dyes.
Properties of Direct Dyes
- They are water-soluble and anionic.
- The dyeing process requires electrolytes and alkaline conditions.
- Generally, cellulosic fiber is dyed, but protein fiber can also be used.
- Fastness properties are average; mainly, the wet fastness is poor. For improved fastness, after-treatment is required.
- They are attached to the fiber with weak hydrogen bonds and Van der Waals’ force of attraction.
- Generally cheap when compared to other dyes.
Primary Mechanism of Direct Dyes
The dyeing involves three steps, adsorption, diffusion, and migration. In water, the cellulosic fiber’s surface becomes anionic. As the dye is also anionic, for neutralizing the surface to remove the repulsion electrolyte is necessary.
Due to swelling in water, the pores of cellulosic fiber’s amorphous region get opened, and the dyes get diffused inside the pores. Dyes are fixed in the fiber with weak H-bond and Van der Waals force. So the fastness properties are not good enough.
Application: Cotton and Viscose.
Reactive Dyes
Reactive dyes have a halogen-containing reactive group present in their structure and become an integral part of the fiber structure by creating a covalent bond. This is the type that is mostly used for dyeing cotton fabrics.
Properties of Reactive Dyes
- These are water-soluble and anionic
- Exhibit good wash and lightfastness and moderate rubbing fastness
- Get fixed into the fiber by a covalent bond
- They are found in powder, liquid, and paste form
- Dyeing requires alkaline conditions and the use of electrolytes.
Primary Mechanism of Reactive Dyes

The primary dyeing mechanism consists of three stages.
- Exhaustion: With the help of electrolytes.
- Fixation: With the help of alkali.
- Washing-off: With the help of a soaping agent.
They generally form two types of reaction with cellulose.
Nucleophilic Substitution Reaction
Cell-OH + HO– ⇒ Cell-O– + H2O
Cell-O– + Dye-Cl ⇒ Cell-O-Dye + Cl–
Nucleophilic Addition Reaction
Cell-O– + Dye-SO2-CH=CH2 ⇒ Dye-SO2-CH=CH2-O-Cell
Application: Mainly cotton, but protein and polyamide can also be dyed.
Acid Dyes
The application of acid dyes requires an acidic bath. They are mainly carboxylic or sulphuric acid salts. These are the types that are mainly used for dyeing protein fibers.
Properties of Acid Dyes
- They are highly water-soluble and anionic.
- They have substantivity towards polyamide and protein fibers.
- They mainly create ionic bonds, but van der Waals and H-bond also contribute.
- The active colored component is the dye anion.
- They show poor wash fastness, but lightfastness is excellent.
- Acidic medium is required for their application.
Primary Mechanism of Acid Dyes
Wool can be represented as,
H2N—W—COOH
In water, under a particular condition,
H2N—W—COOH ⇒ H3N(+)—W—COO(–)
When acid is added,
HCl ⇒ H+ + Cl–
H3N+—W—COO– +H+ + Cl– ⇒ Cl–+H3N—W—COOH
After the addition of dyes with wool, the following reaction takes place:
H3N+−Cl—W—COOH + R– SO3−+Na ⇒ R– SO3−+H3N—W—COOH + NaCl
Wool in Acidic Medium + Acid Dye ⇒ Dyed Wool + Salt
Application: Mainly nylon, silk, and wool.
Basic Dyes
They are mainly salts of organic bases. The cationic part of the dye structure is responsible for color production.
Properties of Basic Dyes
- They are cationic dyes.
- Soluble in alcohol and methylated spirit but not readily soluble in water.
- They exhibit excellent shades but inferior leveling properties.
- They are mainly applicable to jute and acrylic.
- They show average to good fastness properties.
Primary Mechanism of Basic Dyes
The dyeing of acrylic fiber with these dyes is carried out in a weakly acidic condition where acidic acid and sodium acetate is used with a wetting agent, dispersing agent, and sequestering agent. The temperature and pH should be strictly maintained to prevent unlevel dyeing.
The initial uptake of dyes is very high, so a retarding agent is necessary.
Generally, dyeing is carried out at 98°c, and slow cooling is necessary to prevent the formation of creases. Finally, after-treatment with a non-ionic detergent, acetic acid, and a softening agent is carried out.
Application: Best for dyeing acrylic yarn and fabric; can be used to dye jute also.
Vat Dyes
These dyes generally consist of a keto group in their structure and made water-soluble by vatting. Their application process of vat dyes is quite similar to sulfur dyes. They are mainly used for dyeing denim or jeans.
Properties of Vat Dyes
- They are natural coloring substances and not soluble in water.
- Vatting is required to convert them into a water-soluble form, and vatting require alkaline conditions.
- They produce a wide range of colors and brilliant shades.
- They exhibit excellent fastness properties except for the rubbing fastness.
- They are found in powder and paste form.
- They are very expensive, and their application is limited.
Primary Mechanism of Vat Dyes
The dyeing contains four main stages –
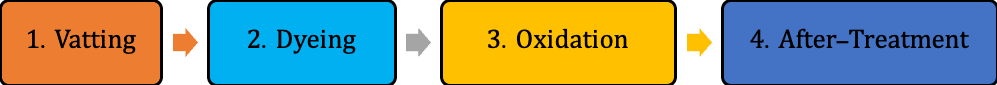
- Vatting: The insoluble dyes are converted into soluble leuco forms by reduction.
- Diffusion: The soluble leuco dyes get inside the cellulosic fiber.
- Oxidation: The soluble dyes converted into insoluble form again.
- Washing-off: The unfixed dyes are removed from the surface.
Application: Cotton.
Sulfur Dyes
Sulfur dyes are quite similar to vat dyes. They are hugely used for producing black and brown shades in cotton. They have a disulfide linkage in their structure.
Properties of Sulfur Dyes
- They are water-insoluble, and the use of reducing agents is required for making them soluble.
- Applied in alkaline condition and oxidation is required for color production.
- Electrolytes can improve the exhaustion.
- They show average fastness properties.
- Range of shades are limited, and generally dull shades are produced.
- They are cheap.
Primary Mechanism of Sulfur Dyes
In order to dye with sulfur dyes, you have to follow the following route –
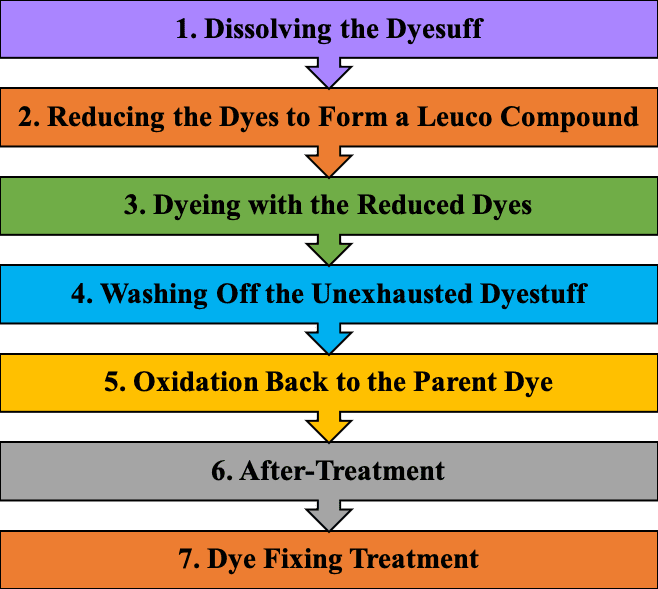
Application: Cotton and viscose.
Disperse Dyes
Disperse dyes are sparingly water-soluble dyes used for dyeing hydrophobic thermoplastic fibers. These synthetic fabric dyes are mainly substituted azo, anthraquinone, or diphenylamine compounds.
Properties of Disperse Dyes
- They have low molecular weight, are non-ionic, and sparingly water-soluble.
- The dispersing agent is required for making dispersed in water.
- They sublime without decomposition and show gas fume fading.
- They are found in powder and liquid forms.
- They have no strong solubilizing group present in their structurer.
- They do not have an affinity towards any fiber.
- They are physically trapped inside the pores of polyester.
Primary Mechanism of Disperse Dyes
The dyeing of Man-Made Fiber (MMF) with disperse dyes follows these steps –
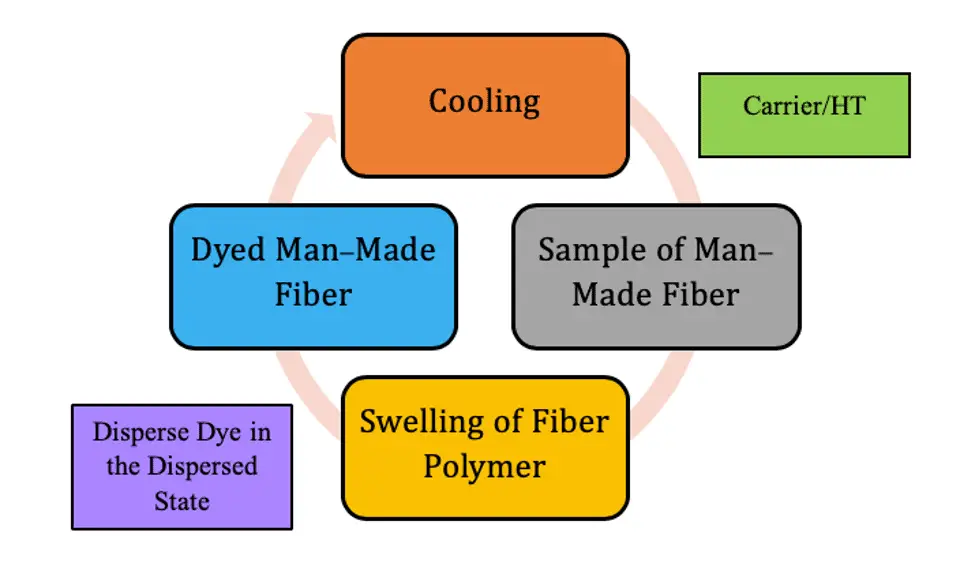
- Dissolution or dispersion of the dye molecules in water
- Adsorption
- Diffusion of the dye molecules in the fiber interior.
Application: Acetate, triacetate, nylon, polyester, acrylic.
Azoic Dyes
They are mainly mono or bis azo water-insoluble coloring compounds. They require coupling components for producing colors. The most exciting thing is that they are not ready-made colors like other dyes.
Properties of Azoic Dyes
- They have an insoluble azo group present in their structure and are not water-soluble.
- Two baths, a developing bath, and an impregnation bath are required.
- They produce intense and bright orange, red and scarlet shades.
- They exhibit excellent washing and lightfastness.
- Their actual color depends on diazonium and coupling compounds.
- They are cheap.
Primary Mechanism of Azoic Dyes
They require two main components.
- Coupling component (Naphthol)
- Diazonium salt
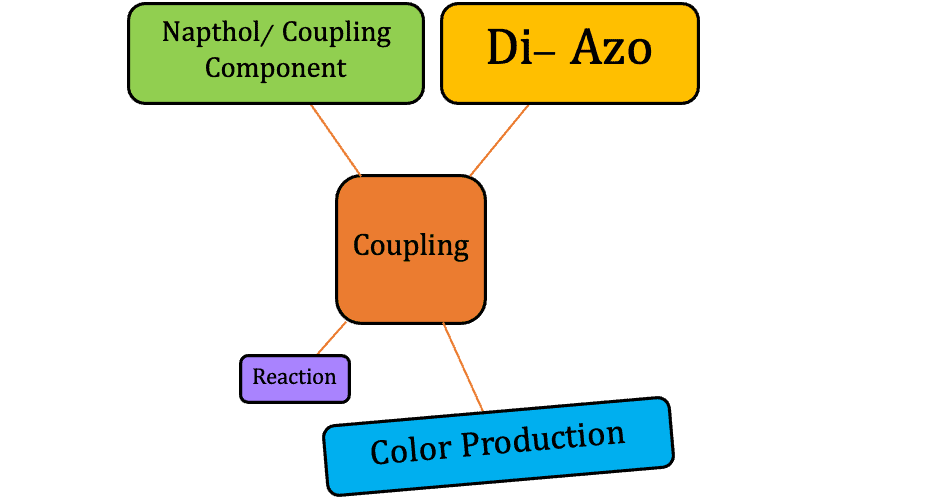
The necessary steps of dyeing are,
- Naphtholation: The insoluble naphthol is made water-soluble by alkali.
- Diazotization: Sodium nitrate reacts with a base containing an amino group that produces diazonium chloride of the base.
- Coupling: The material impregnated in the naphthol solution is added to the diazonium bath to produce color.
Application: Cotton, nylon, polyester.
Mordant Dyes
These dyes have no substantivity towards textile material. Chemical binding agents called mordants help them to get attached to the fiber. They are also called chrome dyes, as they are mainly inorganic chromium.
Properties of Mordant Dyes
- They do not have an affinity for textile material.
- They can produce dark shades.
- They exhibit good fastness properties and leveling properties.
- Dye fiber bond is strong ionic bonds.
- They have a metal ion present in their structure.
- They are soluble in cold water.
Primary Mechanism of Mordant Dyes
A complex is formed with the dyes by the chromium ion when potassium dichromate is added to the solution of acid mordant dyes in the presence of H2SO4. The complex is water-insoluble and gets precipitated in the fiber.
Three processes can be followed.
- Pre-mordanting also known as Chrome
- Meta mordanting also known as Metachrome
- Post mordanting also known as Afterchrome
Application: Natural protein fibers, nylon, and modacrylic fibers.
Summary of the Property & Uses of Dyes
Before finishing this article let’s summarize the most important attributes of these dyes –
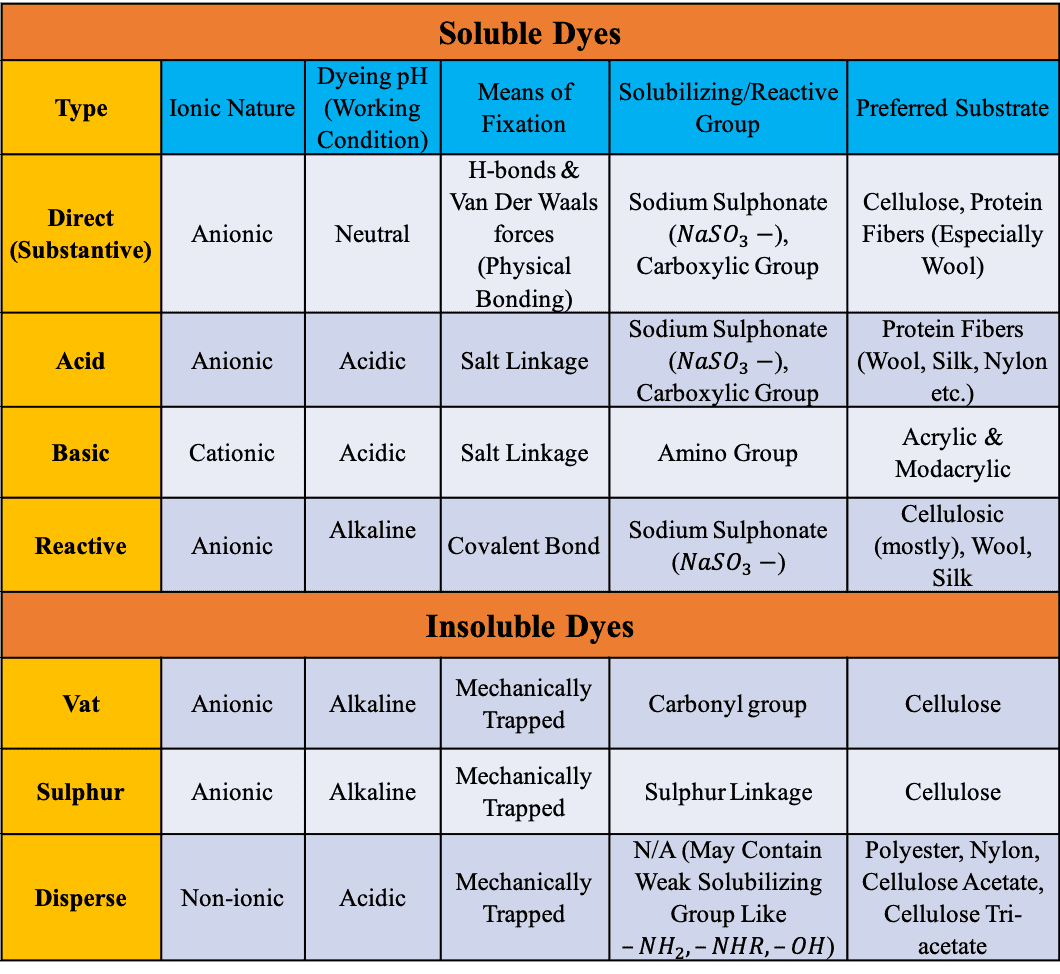
|
Soluble Dyes |
|||||
|
Types of Dyes |
Ionic Nature |
Dyeing pH (Working Condition) |
Means of Fixation |
Solubilizing/Reactive Group |
Uses of Dyes |
|
Direct (Substantive) |
Anionic |
Neutral |
H-bonds & Van Der Waals forces (Physical Bonding) |
Sodium Sulphonate (NaSO3–), Carboxylic Group |
Cellulose, Protein Fibers (Especially Wool) |
|
Acid |
Anionic |
Acidic |
Salt Linkage |
Sodium Sulphonate (NaSO3–), Carboxylic Group |
Protein Fibers (Wool, Silk, Nylon etc.) |
|
Basic |
Cationic |
Acidic |
Salt Linkage |
Amino Group |
Acrylic & Modacrylic |
|
Reactive |
Anionic |
Alkaline |
Covalent Bond |
Sodium Sulphonate (NaSO3–) |
Cellulosic (mostly), Wool, Silk |
|
Insoluble Dyes |
|||||
|
Vat |
Anionic |
Alkaline |
Mechanically Trapped |
Carbonyl group |
Cellulose |
|
Sulfur |
Anionic |
Alkaline |
Mechanically Trapped |
Sulfur Linkage |
Cellulose |
|
Disperse |
Non-ionic |
Acidic |
Mechanically Trapped |
N/A (May Contain Weak Solubilizing Group Like –NH2,–NHR, –OH) |
Polyester, Nylon, Cellulose Acetate, Cellulose Tri-acetate |
Direct Dye vs Reactive Dye
Here’s the difference between direct dye and reactive dye –
| Property | Direct Dye | Reactive Dye |
|---|---|---|
| Type of Bond | No chemical bonds, just Van der Waals force | Covalent bond |
| Dyeing Condition | Basic | Acidic |
| Colorfastness to Wash | Very poor | Excellent |
| Mostly Used for | Cotton & Viscose | Cotton & Polyamides (sometimes) |
| Example | Direct Black E | CI Reactive Blue 19 |
Vat Dye vs Reactive Dye
Here are the most obvious differences between vat dye and reactive dye –
| Properties | Reactive Dyes | Vat Dyes |
|---|---|---|
| Preparatory Process | Reactive dyes don’t require any sort of extra preparatory processes. | Vat dyes require vatting. |
| Dyeing Container Condition | Doesn’t require to be dyed in air-tight conditions | Requires to be dyed in air-tight conditions |
| Possibility of Hydrolysis | Prone to hydrolysis, thus huge amount of dye wastage | Prone to over-reduction, needs ORP value monitoring |
| Fastness to Cross-staining | Cross-staining fastness isn’t as good as vat dyes. | Excellent fastness to cross-staining |
| Cost | Comparatively less expensive than vat dyes | One of the most expensive dyestuff |
| Example | CI Reactive Blue 19 | CI Vat Red 10 |
Reactive Dye vs Disperse Dye
The difference between reactive and disperse dyes is that –
| Properties | Reactive Dyes | Disperse Dyes |
|---|---|---|
| Solubility | Water-soluble dyes | Initially insoluble, have to make soluble using a dispersing agent |
| Dye-Fiber Bond Type | Covalent Bond | Mechanically trapped inside the fiber |
| Dyeing Container Temperature | Commercially, mostly dyed at 60°C or around boil | High-Temp-High-Pressure (HTHP) machines are required to reach 135°C |
| Possibility of Hydrolysis | Prone to hydrolysis, thus huge amount of dye wastage | No hydrolysis, lesser dye wastage |
| Fastness to Wash | Excellent | Excellent |
| Mostly Used for | Natural fabrics | Synthetic fabrics |
| Example | CI Reactive Blue 19 | Disperse Blue 6 |
Frequently Asked Questions
1. Which types of colorants have the best wash fastness?
It varies depending on the material. For example, both are reactive dyes, and vat dyes have excellent wash fasteners on cellulosic fibers as well as cotton and tetron. However, their dyeing procedure is very different.
2. What is the most suitable kind of dyes for dyeing cotton?
That would be fiber reactive dyes. They give a wide variety of shades, possess excellent wash fastness and comes at a cheaper price too.
3. What are the best types of dyes for dyeing polyester?
When it comes to polyester, disperse dyes should be your go-to choice. They require a higher dyeing temperature, though. We have a detailed article on it. Give it a read to know more.
REFERENCES
- Introduction to textile engineering by Dr. Hosne Ara Begum
- Houser, Textile dyeing: InTech, Croatia, 2011
- Encyclopedia Britannica: Dyeing and Printing
- Basic principles of textile coloration by A.D. Broadbent
- Textile preparation and dyeing by A.K.R. Choudhury
- Fundamentals and practices in coloration by J.N. Chakraborty

