Acid Dyes -Types | Properties | Dyeing Mechanism
Dyeing protein fibres with acid dyes is a chllenging task itself, since the mode of fixation is different. When you add the fact that water acts as a lubricator for fibres like nylon, the problem gets even more complicated.
Anyway, this article will provide you a brief look into acid dyes. Let’s get started –
Why It’s Called Acid Dyes?
Acid dyes are mostly sulphuric, or carboxylic acid salts and are essentially applied from an acidic bath and hence the name ‘acid dye.’
Mechanism: Acid Dyeing of Wool
Based on the chemical structure of wool, it may be simply represented as H2N –W–COOH, where ‘W’ denotes the main non-reacting body of the wool structure. When wool is immersed in water, the following reaction takes place –
H2N-W-COOH + H2O →H3N+-W-COO−
Now, when acid is added –
H3N+-W-COO−+ CH3COOH → H3N+−CH3COO-W-COOH
After the addition of dyes –
H3N+−CH3COO-W-COOH + R– SO3−+Na → R– SO3−+H3N-W-COOH + CH3COONa
Classification of Acid Dyes
Based on chemical nature, acid dye can be classified as –
- Monoazo and Bisazo
- Nitro
- Nitroso
- Triphenylmethane
- Xanthene
- Azine
- Quinoline
- Ketonimine
- Anthraquinone
- Phthalocyanine
Here you need to remember that, for azo dyes,
- The substantivity of the dyes will increase if it has higher molecular weight
- If the number of sulphonate group per dye molecules decreases, the substantivity will also increase
Classification of Acid Dyes According to Dyeing Characteristics
Based on dyeing characteristics (wool dyeing) acid dye can be classified in,
- Levelling acid dye
- Fast acid dye
- Milling acid dye
- Super milling acid dye
Characteristics of the Various Types of Acid Dyes
| Parameters | Levelling | Fast | Milling | Super Milling |
|---|---|---|---|---|
| Acid used | Sulphuric | Acetic | Acetic or NH4+ | NH4+ |
| Dyebath pH | 2-4 | 4-6 | 5-7 | 6-7 |
| Migration ability | High | Moderate | Low | Very low |
| Washing fastness | Poor-fair | Good | Very good | Very good |
| Molecular weight | Low | Moderate | High | Very high |
| Dye solubility | High | Moderate | Low | Low |
| State in solution | Molecular | Aggregated | Aggregated | Aggregated |
| Substantivity (pH 6) | Very low | Moderate | High | High |
Classification of Acid Dyes for Nylon Dyeing
I. Group A
This includes dyes with good affinity in weak acid liquor, good migration and levelling properties.
II. Group B
This includes acid dyes with good affinity in neutral liquor. They have higher rate of dyeing but less migration than group A.
Which Dye Will You Choose at Which Situation?
As there are different type of acid dyes available, it’s a very reasonable question. The simple answer is that, the selection should be based on fastness and levelness requirements.
Strong acid dyes easily develop well levelled shades with poor wash fastness, while milling and super-milling dyes produce fast shades.
If fastness is to be improved in post-dyeing stage through after-treatment with metal salts, dyes with chelating sites are preferred.
Acid Dyeing Parameters
Different types of dyes require the employment of different kinds of parameters. These parameters have to be maintained at an optimal level to attain a satisfactory dyeing result. For acid dyes, there are 3 parameters that are of utmost importance. Let’s take a look.
1. Effect of Electrolyte
Electrolytes in acid dyeing act as levelling or retarding agent. Due to opposite electrical nature of dye and fibre, strike rate and uptake of acid dye is faster.
Also, the electrolytes slow down the initial rate of dyeing by creating a temporary bond with the dye or fibre which disassociates when the temperature is raised. Thus, electrolyte prevent unlevel dyeing.
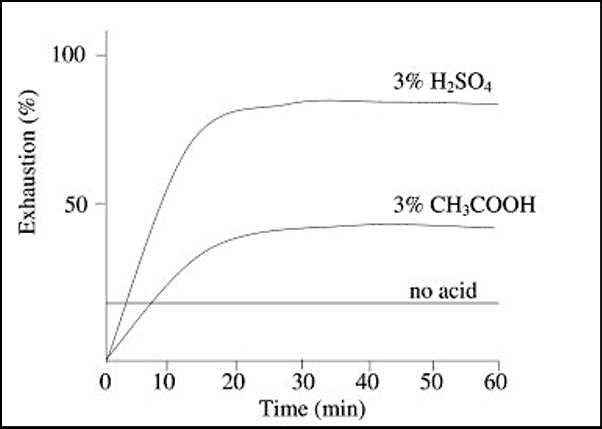
2. Effect of Acid
Not only the total amount of the dye adsorbed is influenced by amount of acid, but the rate of exhaustion is also dependent upon acidity or pH of bath.
3. Effect of Temperature
Acid dyes added to bath can attach themselves with cationic site of fibre through the replacement of anion released by acid. And it is not possible at room temperature, rather only when the bath is heated up, causing acceleration of dye molecules to generate the required momentum.
Excellent dyeing results are obtained if dyeing is started at about 40°C, raised slowly to boil and dyeing is further carried out at boil for desired time. Acid dyes are not transferred from bath to fibre below 39°C.
Milling acid dyes have a minimum temperature of exhaustion at 60°C, but at 70°C, transfer of dye is fast. Super-milling dyes cause level dyeing only at boil.
Properties of Acid Dyes
- Acid dyes are usually sodium salts of sulphonic acids, or less frequently of carboxylic acids, and are therefore anionic in aqueous solution.
- They have substantivity towards protein and polyamide fibres.
- These dyes are suitable for wool, silk, polyamide and modified acrylics.
- These dyes have no affinity for cotton cellulose, hence not suitable for cellulosic fbres.
- They are highly water soluble.
- Acid dyes have molecular weights in the range 300–1000 g mol-1.
- They create ionic bonds with fibre, hydrogen bond and Vander waals force of attraction also contributes.
- The dye anion is the active colored component in these dyes.
- Overall wash fastness of acid dye is poor, though light fastness is quite good.
What Can You Do to Improve the Wash Fastness of Acid Dyes?
For improving the wash fastness of nylon dyed with acid dyes, back tanning is done. This treatment is required for bathing suits or multi colored fabrics. This process produces a film on the surface of the fibre that hinders the detachment of the dye from the fibre. Two types of back tanning are possible.
Full Back Tanning
Treatment with liquor containing 2% tannin and 2% acetic acid for 20 minutes at 70°c temperature. When all the tannin is absorbed, 1% Tartar emetic is added and treatment is carried out for further 30 minutes.
Half Back Tanning
Treatment with Syntan is called half back tanning as it does not show wash fastness properties as high as full back tanning.
Comparison of Acid Dye Application on Wool, Nylon, and Silk
Wool
- Wool fibre contains a large number of amino groups. There are approximately twenty times as many amino groups on wool when compared to nylon and five times as many amino groups when compared to silk.
- As wool is highly amorphous in nature, dark shades can be easily be obtained while dyeing wool sweaters.
Nylon
- The dyeing properties of nylon with acid dyes are similar to wool. The shades are very similar for nylon but the saturation point is lower.
- As nylon is more crystalline than wool and have a smaller number of amino groups, darker shades can’t be obtained.
Silk
- Silk has an affinity for acid dyes but the colors tend to be less fast when compared with wool.
- Silk exerts its affinity for acid dyes at lower temperature than wool, and dyeing is usually carried out at 40ºC and the maximum temperature is limited to 85ºC.
- Glauber’s salt is used silk as it diminishes its luster.
- As strong acid such as sulphuric acid damages the silk, acetic acid is used.
REFERENCE

