What is Nylon – Properties of Nylon and Its Uses
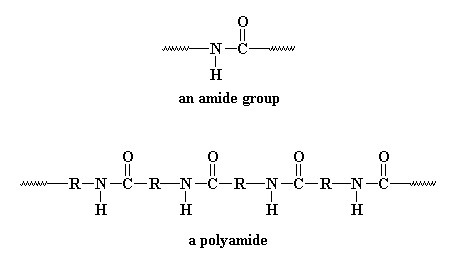
Polyamides
Polyamides are polymers that contain an amide group (-CONH-) in it’s backbone as a recurring part of the chain. They are frequently referred to as Nylon which has thermoplastic silky properties.
Nylon
When less than 85% of amide linkage are attached directly to two aliphatic groups the polyamides are known as Nylon.
Aramids are fibers of aromatic polyamides where at least 85% of the amide linkages are attached directly to two aromatic rings.
We’ll discuss about aramids in another post.
Invention

- Nylon was the first commercially successful synthetic polymer
- Wallace Carothers is credited with the invention of synthetic rubber and nylon around 1933 at DuPont.
- It was first produced on February 28, 1935 at the DuPont Experimental Station
- Fiber went commercial around 1938 and is still used extensively today.
- DuPont recouped all investment in Nylon 6,6 within 30 days of plant startup as there had been nothing like it before !!!
Nomenclature
Nylons are formed mainly by condensation polymerization though they can also be formed by addition polymerization .
It can be formed by 3 approaches –
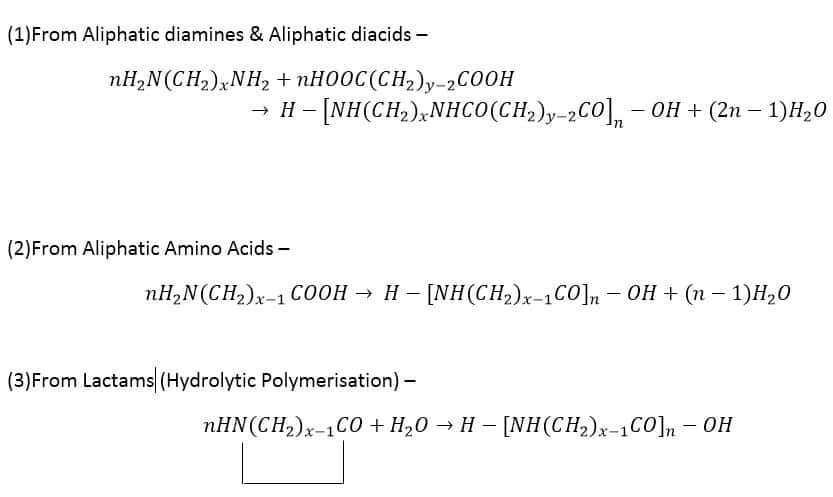
Nylon 6,6
It’s made from Adipic acid and Hexamethylene diamine. Both of these monomers contain 6 carbon atoms hence the name Nylon 6,6. Before the name Nylon 6,6 was used, it was called by the codename “fiber 66”
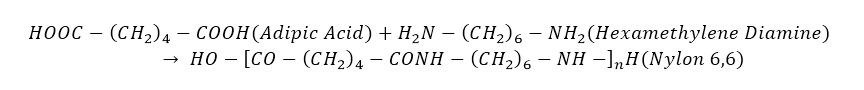
Nylon 6
In 1938 Paul Schlack of the IG Farben Company in Germany polymerized Caprolactum & created a different form of the polymer identified as Nylon 6.
It’s a homopolymer which is formed by ring opening polymerisation.
The Degree of Polymerisaton for Nylon 6 & Nylon 6,6 ranges between 100 to 180 & their Average Molecular Weight ranges between 15,000 to 30,000
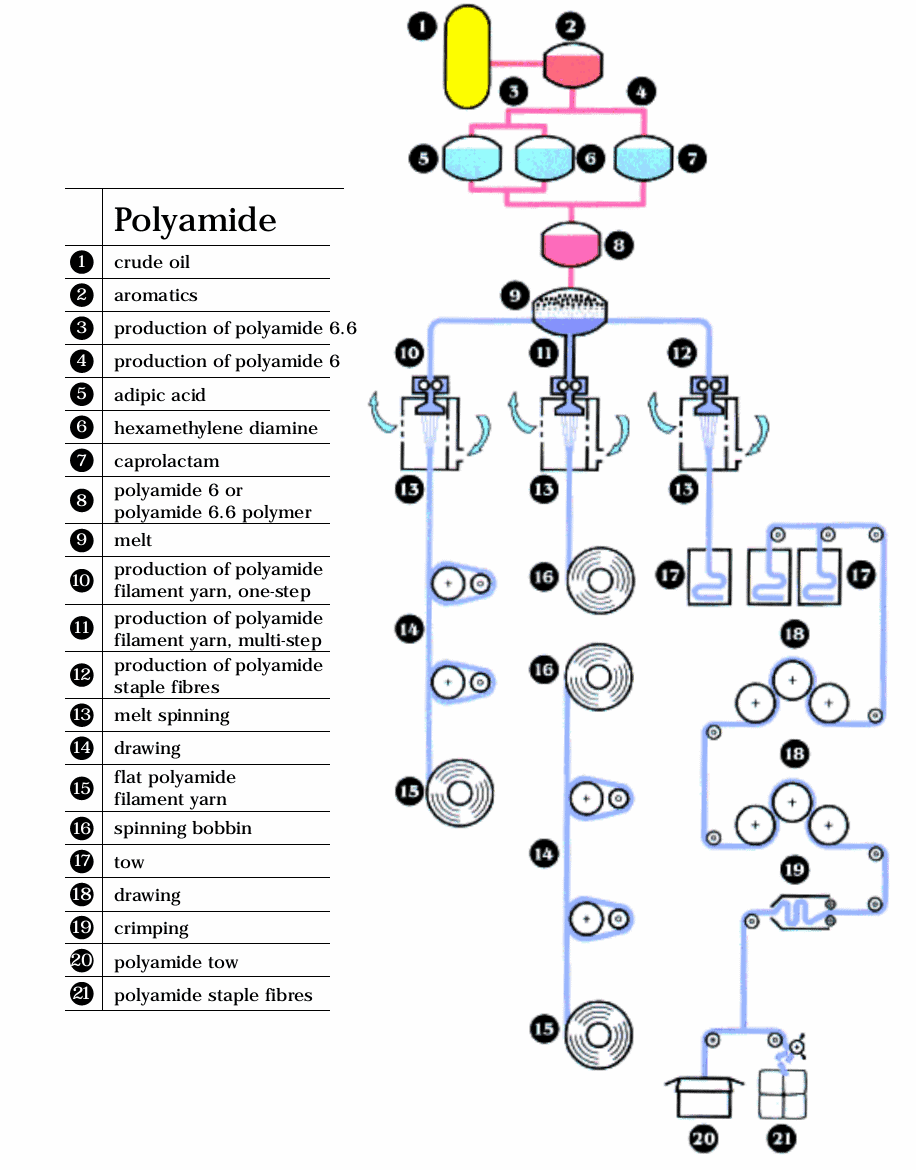
Spinning Process Diagram
Chemical Properties
Swelling of Nylon
The Oxygen of the Carbonyl group is slightly negative & the hydrogen (imino hydrogen) is slightly of positive charge.The polar group in Nylon and is responsible for swelling in water or in polar solvents or in dyeing with disperse & metallized dyes.
Melting of Nylon
Melting point changes because of the following reasons –
The increase of CONH group to CH2 groups
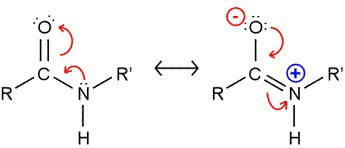
The amide group is planar in nature because of the partial double bond character of C-N bond. It has been estimated that the barrier for rotation about this bond may be 63 Kj/mol (15 Kcal/mol) or higher.For this reason if the ratio of CONH group to CH2 is high than melting temperature would be high also.
Hydrogen Bond
The polar polyamide group is responsible for hydrogen bonding between polyamide chains. Whether the no. of CH2 groups between CONH is odd or even is a very important factor.
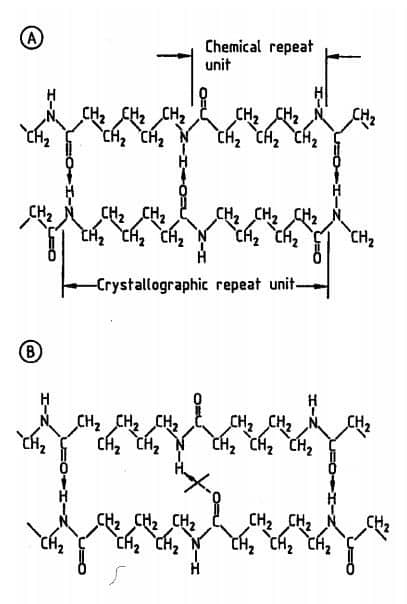
If the no. of CH2 group between amide groups is odd & the orientation of the chain is anti-parallel/opposed (Fig. A) then it allows complete hydrogen bonding.
But if the orientation of the chains is parallel/same the bonding is not complete. Now changing from a parallel array to an anti-parallel array requires the inverting of the whole chain in case of odd no. But only a segmental lateral movement is needed if the no. of the CH2 group is even (Ex. Nylon 6,6).
So the Nylon 6 polymer has a lower melting point than Nylon 6,6.
Based on this we can say that the polyamide having odd no. of CH2 group will have lower melting temperature that the similar kind of polyamide having a even no. of CH2 group
Introduction of Side Chains
If side chains are introduced into the carbon skeleton then it interferes with the intermolecular forces between the amide groups.It results in reduction of melting point and increases solubility in organic solvents.
Introduction of Aromatic Ring
when the amide group is connected to aromatic rings it results in chain stiffening and higher melting temperature. This class of polyamides are called aramids which usually degrades without melting so it can’t be melt spun.
Final Words
Nylon is a polymer made of repeating amide monomer units. Its valuable properties, like strength, resilience, and flexibility, have made it an excellent choice for various applications, from textiles to engineering plastics. For example, nylon was the primary choice for athletic wear until Spandex was introduced.
For many years, nylon was the primary choice for athletes’ clothing. However, in recent years, Spandex has become increasingly popular. Nylon and Spandex are both prized for their flexibility and comfort, making them ideal for athletes who want to perform at their best.
References
- Apparel Fibers – Dr. Engr. Md. Nazirul Islam
- Industrial Polymers Handbook –Edward S. Wilks
- Man Made Fibres –Cesare Andreoli & Fabrizio Freti
Images:

