Heat of Wetting (An Exceptional Property of Wool)
Heat of Wetting
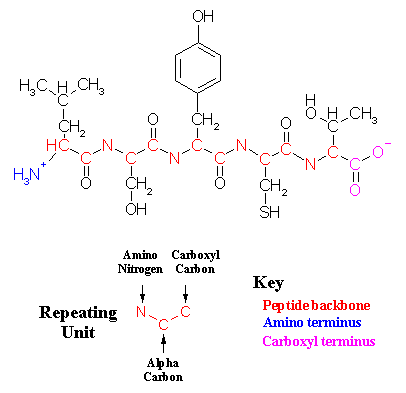
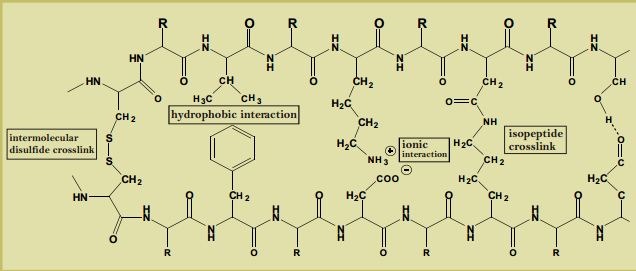
Wool fiber can soak a lot of moisture (up to 30% of its weight) without getting wet or clammy.This is called heat of wetting. The hydroscopic polar groups or the side chains (eg. Hydroxyl) on the polypeptide chain associates the process, although wool fiber has water repellent epicuticle membrane (3-6nm) on its surface. The protein matrix containing isopeptide cross linkage makes epicuticle remarkably chemical resistant.
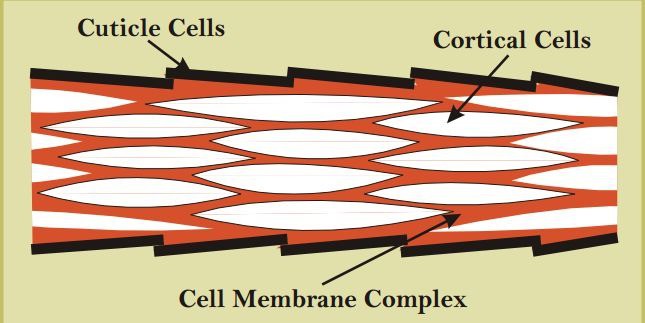
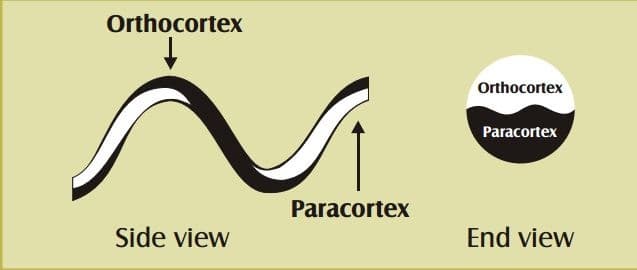
Underneath the epiuticle there lie two layers, orthocortex and paracortex. The first one is elastic and flexible while the other one is stable and hard because of more cystine (cystine is a sulphur having amino groups that make disulphide linkage) on it. The comparative hardening or asymmetrical growth of these two cortical layers causes crimp on wool fiber. Crimp holds the fiber strands together opposing them to line side by side or lay down flat, so the tiny air pockets remain intact.
Here are some images to illustrate the concept clearly –
Mechanism
- When fiber absorbs moisture the hydrogen bonds of water break down as water reacts an exothermic reaction with the polar groups of polypeptide chain liberating heat that gets trapped between the air pockets.
- The air can’t move or circulate on those pockets and heat transfer doesn’t occur by convection.
- The air pockets allow moisture to evaporate and make sure one doesn’t get overheated while sweating. This cooling occurs gradually
- When the weather becomes drier the polar groups of polypeptide chain release water and energies are retained in the environment by an endothermic reaction, though it cant release adequate vapors to maintain equilibrium.
Heat of Wetting Calculated for Some Fibres
| Wool | 113 J/g |
| Viscose | 106 J/g |
| Silk | 69 J/g |
| Flax | 55 J/g |
| Cotton | 46 J/g |
Actually wool absorbs moisture both from body and environment, and makes a dry warm condition.
REFERENCES
- Handbook of textile fibres (J. Gordon Cook)
- Apparel fibers (Dr. Eng. Md. Nazirul Islam)
Images:
http://www.spletnik.ru/blogs/moda/46138_tkani_naturalnyenaturalnye