How Does Soap Clean Clothes?
Detergency
The compounds which can remove dirt/oil from material & keep it in solution by the means of suspension are called detergents. This property is called detergency.
It’s a property of a compound that is closely related to surface tension.
A satisfactory detergent must possess the following properties:
(1) Good wetting characteristics in order that the detergent may come into intimate contact with the surface to be cleaned.
(2)Ability to remove or to help remove dirt into the bulk of the liquid.
(3)Ability to solubilize or disperse removed dirt and prevent it from being re-deposited onto the cleaned surface.
Read this post to learn about Soap & Micelle
As we learned earlier soap molecules have a hydrophilic head (water-seeking) & hydrophobic tail (water-repelling), it won’t be distributed in the water uniformly rather the hydrophobic tail would orient itself to the surfaces.
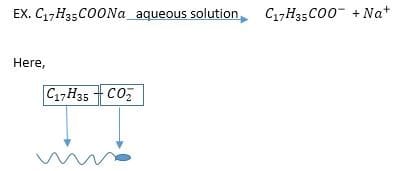
(The long alkaline chain is hydrophobic and the carboxylate ion is the hydrophilic part)
At the interfaces (ex. fabric-water, oil-water), the long alkyl chain tends to move away from the water phase as a result surface tension decreases because the force acts opposite to the inward pull of water molecules. So, we can say that soap is a surface-active compound that reduces the surface tension of water.
Let’s consider an oil drop on a fabric –
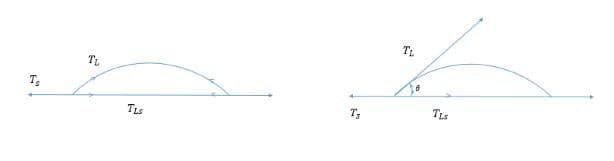
The shape of the oil drop will depend on the following 3 forces:
TL =The surface tension between the oil drop and the surrounding aqueous phase
TS = The surface tension between the fabric & surrounding aqueous phase
TLS = The surface tension between the fabric and oil drop
We can see that if TLS + TL increases in relation to TS the area of contact between oil and fiber will decrease. This will lead the shape of the oil to be like a sphere with a decreasing contact angle (θ)
When the forces are in equilibrium, their relationship is expressed by Young’s equation neglecting the gravity effect:
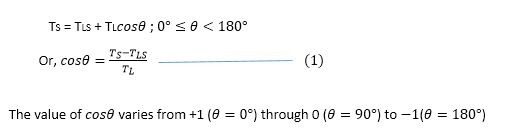
Here, 3 cases are considerable-
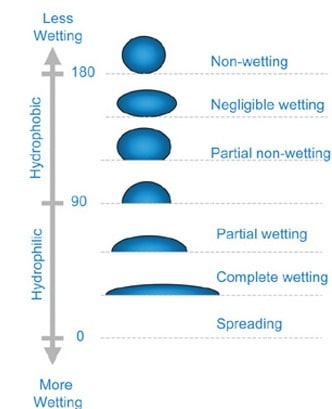
Hydrophobic Impurities
Here are two images to understand the concept clearly –
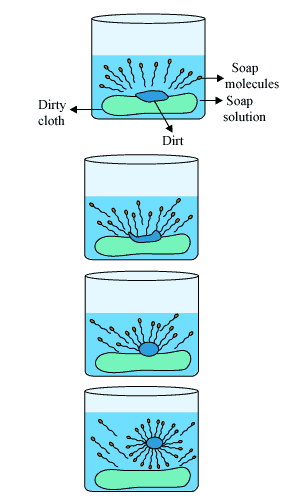
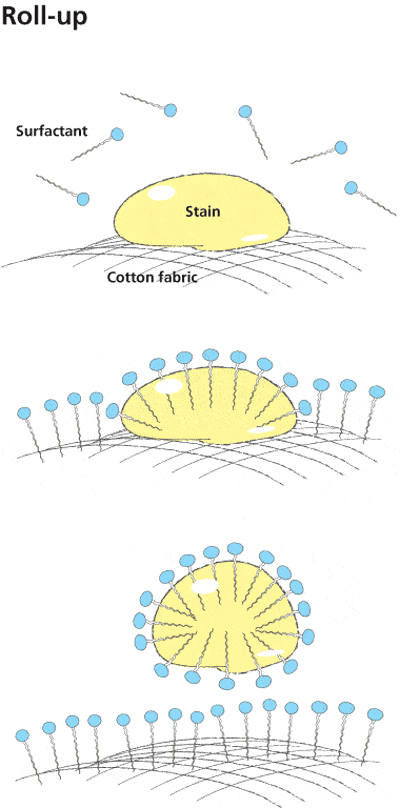
- The Hydrophobic tails saturate the dirt/oil & fabric.
- Fiber-water & oil-water surface tension decreases.
- The surface tension between fiber and oil is unchanged so from equation (1) we will get a negative value of cos which means the contact angle is now more than 90
- This unchanged surface tension between fiber and oil & repulsion between the negative charges of fiber water and oil-water interface will cause the oil to reduce its surface area by rolling up
- The oil begins to lift from the fiber & is removed into the solution where it’s suspended by electrostatic repulsion.
- The oil/dirt won’t be re-deposited because of the negatively charged repulsion between oil/dirt water & fiber water interfaces
Hydrophilic (Polar) Impurities
- The hydrophilic head orients itself to the polar dirt
- A second layer is formed where the hydrophobic tail orients itself to the first layer of tails
- The rest of the mechanism is similar to the previous system
References:
Books:
- Dyeing and Chemical Technology of Textile Fibres by E.R. Trotman
- Textile Preaparation & Dyeing by A K Roy Choudhury
Images:

