Reduction Potential of Vat Dyeing | ORP Values of Reducing Agents & Their Properties
First things first, we need to address why do we need to measure the reduction potential or ORP value of the vatting bath.
You see, we need to ensure the appropriate vatting condition in which all the vat dyes will get reduced. And you already know that if the dyes don’t get reduced, it won’t be soluble. So, we must use a quantative measure to ensure the reproducibilty of the vatting condition.
If we monitor the ORP value of the bath, then we will be able to adjust the concentration of the reducing agent accordingly. Therefore, the loss in reducing agent due to the presence of air will be made up for.
Furthermore, we’ll be able to maintain a perfect ORP value in which the dye will stay in its reduced form.
What is Reduction Potential (ORP)?
Reduction/oxidation potential or redox potential (mV) is a measure of the tendency of a chemical species to be reduced and thereby acquire electrons.
When a given compound possesses a particular redox potential—the more negative the potential, the greater the tendency of that compound to be reduced (i.e. to acquire electrons).
Thus, reducing agents have negative redox potentials whereas oxidising agents have positive redox potentials.

What Reduction Potential or ORP Value Vat Dyes Require?
Vat dyes generally display redox potentials in aqueous media in the range of –770 to –1000 mV, the majority being in the region of –900 to –950 mV.
However, for indigo, it is -600 mV.
What Are Reducing Agents?
Reducing agents are compounds which either donate hydrogen to, subtract oxygen from or add electrons (negative charges) to other chemicals. The affected chemicals are said to be reduced. During the reduction process, the reducing agent itself is changed (oxidized), often irreversibly.
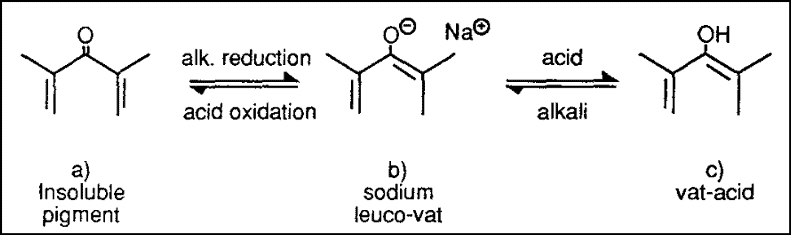
For example, the vat pigment structure in the above figure (a) is reduced to give the structures shown in figure (b):the soluble sodium leuco-vat. Or it can produce the sparingly soluble leuco vat-acid (c), which have an added negative charge and a hydrogen atom respectively.
To be practically useful, solutions of a vat dye reducing agent must have a level of reducing power (reduction potential) sufficient to reduce all commercial vat dyes to their water soluble forms, economically and quickly, without converting the dyes to products from which the original pigments cannot be restored (over reduction).
What Are the Common Reducing Agents Used in Vat Dyeing?
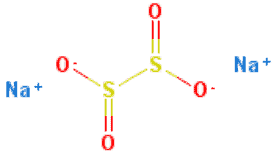
The most widely used reducing agent is sodium hydrosulfite or commonly known as hydros. It is also known as sodium dithionite. We’ll start with it, and then go over the other ones below –
1. Sodium Dithionite / Sodium Hydrosulfite / Hydros
Chemical Names: Sodium dithionite, Sodium hydrosulphite, Sodium sulfoxylate, Disodium dithionite, Vatrolite, Hydros, Dithionous acid.
Molecular Formula: Na2S2O4
Molecular Weight:174.11 g/mol (anhydrous), 210.146 g/mol (dihydrate).
Properties of Sodium Dithionite / Sodium Hydrosulfite / Hydros As a Reducing Agent in vat Dyeing
- It is the universally accepted reducing agent in vat dyeing.
- Reduces insoluble vat dye to partially soluble leuco dye, counter-acts effect of dissolved oxygen in water.
- The anhydrous form is stable.
- Contact with water readily forms sodium bisulphite and sodium thiosulphate; formation of acidic products accelerates decomposition, which is exothermic and can result in spontaneous ignition.
Na2S2O4 + H2O = Na2S2O3 + 2NaHSO3
- Reduced form is quite stable in presence of NaOH to carry out dyeing at room temperature.
- It gets decomposed in water, thermally, oxidatively, and in various other ways
Precautions Before Using Hydrose As Reducing Agent for Vat Dyes
Little excess of it retains stability of reduced liquor required for levelled dyeing, and produces a sediment free clear reduction bath. However, if used in too excess, it retards the rate of dyeing. And using in huge amounts results in over reduction as well as wastage of dye.
Advantages
- Sufficient reduction potential (-700 to -1000mV) for vat, sulphur or indigo dyeing.
- Good stability of leuco vat dyebaths.
Disadvantages
- Waste water loading; inhibits biological degradation & leads to a greater oxygen demand.
- Can cause over-reduction at higher temperatures.
- Special safe storage facility required.
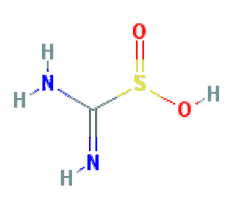
2. Thiourea Dioxide
Chemical Names: Thiourea dioxide; Amino(imino)methanesulfinic acid; Formamidinesulfinic acid.
Molecular Formula: CH4N2O2S
Molecular Weight: 108.12 g/mol
Physical Structure:
Properties of Thiourea Dioxide As a Reducing Agent in vat Dyeing
- Giber higher ORP values than most other reducing agents
- A strong dye-reducing agent, but in alkaline solutions it is just as sensitive to atmospheric oxygen as hydrosulphite
- Its low sulphur content lead to better values for sulphite and sulphate in the effluent
- More prone to oxidation than Sodium Dithionite/ Hydros
- In high temperature process, it is far more liable than Hydros to cause over-reduction
- Highly priced
Advantages
- Sufficient reduction potential for vat, sulphur or indigo dyeing
- Especially suitable for high-temperature dyeing methods
- Good resistance to oxidation by air
Disadvantages
- Waste water loading; inhibits biological degradation & leads to a greater oxygen demand
- Potentially temperature-dependent
3. Sodium Hydroxymethanesulfinate
Chemical Names: Sodium hydroxymethanesulfinate; Rongalite; Rongalite C; Aldanil; Discolite; sodium formaldehydesulfoxylate.
Molecular Formula: CH3NaO3S
Molecular Weight: 118.09 g/mol
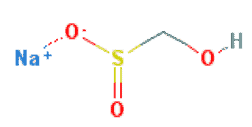
Properties of Sodium Hydroxymethanesulfinate As a Reducing Agent in Vat Dyeing
- Much more stable than sodium dithionite at lower temperatures
- Can be used to prepare stable pad liquors and print pastes
- At higher temperatures, as in steam fixation treatments, it’s capable of bringing about rapid reduction of vat dyes
- It finds use in vat printing and for dyeing at temperatures above 100°C
Advantages
- Sufficient reduction potential for vat, sulphur or indigo dyeing.
- Especially suitable for high-temperature dyeing methods.
- Good resistance to oxidation by air.
Disadvantages
- Waste water loading; inhibits biological degradation & leads to a greater oxygen demand.
- Potentially temperature-dependent.
4. Hydroxyacetone
Chemical Names: Hydroxyacetone; Acetol; 1-Hydroxy-2-propanone; Acetone alcohol.
Molecular Formula: C3H6O2
Molecular Weight: 74.07854 g/mol
Physical Structure:
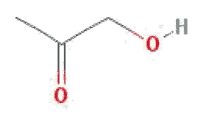
Properties of Hydroxyacetone As a Reducing Agent in Vat Dyeing
- Hydroxyacetone is sulphur-free and biodegradable
- Can be used for the pad–steam application of vat dyes in the presence of high concentrations of sodium hydroxide (about 3.5–4.5 g/l)
- The RD Process (Refine Dyeing process) makes it suitable for applying indigo on continuous yarn-dyeing ranges
- Hydroxyacetone doesn’t cause over-reduction of indanthrone vat dyes
Advantages
- Biologically degradable.
- Results in lower COD values, and the effluent contains no sulphide, sulphite or sulphate
- Ease of dosing in liquid form.
- Very good stability to storage.
Disadvantages
- Does not reach full reduction potential & thus mainly suitable for indigo & sulphur dyes.
- Persistent odor.
- Limited commercial production
5. Sodium Borohydride
Chemical Names: Sodium borohydride; Sodium tetrahydroborate; Borol; Sodium tetrahydridoborate; Sodium borohydrate.
Molecular Formula: NaBH4
Molecular Weight: 33.80 g/mol
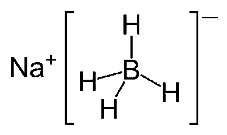
Properties of Sodium Sodium Borohydride As a Reducing Agent in Vat Dyeing
- It is a bi-component reducing system and is suitable for pad – steam process
- Gives high reduction potential, but fails to reduce vat dyes sufficiently.
NaBH4 + 2H2O = NaBO2 + 8H
For the conversion of a vat dye to its leuco form and its maintenance during dyeing, the specificity of the reducing agent is more important than the value of reduction potential of the reducing agent.
- Can be used only along with accelerator (NaCl)
- Fails to stabilize leuco vat dye
- Generally reacts too slowly to be usable in vat dyeing
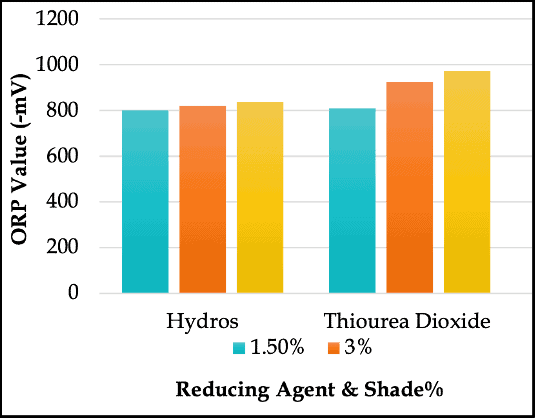
ORP Values of the Reducing Agents Used in Vat Dyeing
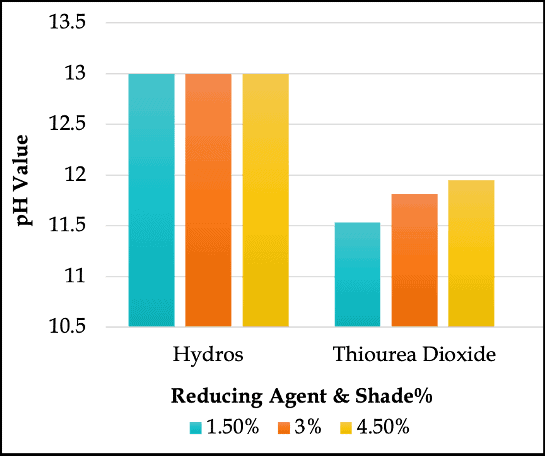
pH Values of the Reducing Agents Used in Vat Dyeing