Reactive Dyes – Classification | Properties | Dyeing Method
What is Reactive Dye?
Reactive dye is a class of dye that makes a covalent bond with the fiber and becomes an integral part of the fiber. These are usually used to dye cellulosic fibers such as cotton, rayon, or flax, but polyamide, wool, silk, and acetate fibers can also be dyed using reactive dyes. Reactive dyes are so-called because this is the only type of dye that has a reactive group. This group reacts chemically with the fiber polymer molecules to form covalent bonds. This covalent bond is formed between the reactive group and terminal –OH group of cellulosic fibers and terminal –NH2 group of polyamide and wool fiber. If we assume the general structure of reactive dye is S–F–T–X, then this can be described as: S represents the chromophore, which gives color to the dye, F is the functional group that helps in bonding, T is the bridging group, and X is the reactive group responsible for forming covalent bonds with the fiber. This unique structure makes reactive dyes versatile and a preferred choice for achieving vibrant, long-lasting colors. Particularly in hand dyeing yarn for beginners, reactive dyes are favored for their ease of use and ability to produce rich, uniform shades on natural fibers like cotton or silk.
S–F–T–X + fibre = S–F–T–X–fibre
Where,
- S = Solubilising groups (such as SO3Na or COONa or combination of both)
- F = Chromophoric group usually an azo, metal-complex azo, anthraquinone, etc.
- T = Bridging group which attaches the reactive system X to the chromogen F; Usually, –NH, –O–, –NHCO–, –OCH3–, –SO3–, etc.
- X = Reactive system or group, which reacts chemically with the functional group of the fibre
Here’s an example of reactive dye with its various parts:
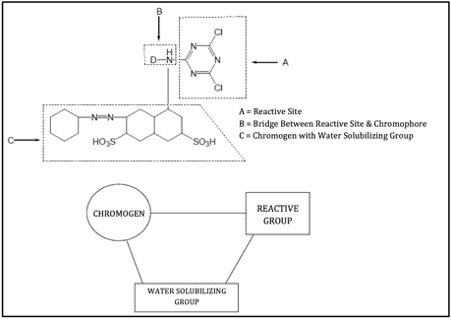
Some Trade Names of Reactive Dyes
| Brand Name | Manufacturer | Country |
|---|---|---|
| Procion | I.C.I. | UK |
| Novacron | Huntsman | Switzerland |
| Remazol | Hoecht | Germany |
| Levafix | Bayer | Germany |
| Reactone | Geigy | Switzerland |
| Primazin | BASF | Germany |
| Drimarine | Sandoz | Switzerland |
Classification of Reactive Dyes
1. On the Basis of Reactive Group
Halogen
- Triazine group: Procion, Cibacron
- pyrimidine group: Reactone
- Quinoxaline group: Levafix
Activated Vinyl Compound
- Vinyl sulphone: Remazol
- Vinyl acrylamide: Primazine
- Vinyl sulphonamide: Levafix
2. On the Basis of Reactivity
- Lower reactive dye: pH is maintained 12-12.5 using NaOH in bath
- Medium reactive dye: pH is maintained 11-12 by Na2CO3
- Higher reactive dye: pH is maintained 10-11 using NaHCO3
3. On the Basis of Dyeing Temperature
Cold Brand Reactive Dyes
These types of dyes contain reactive groups of high reactivity. So dyeing can be done in lower temperature i.e. 32-60°C. For example: PROCION M, LIVAFIX E
Medium Brand Reactive Dyes
This type of dyes contains reactive groups of moderate reactivity. So dyeing is done at higher temperatures than that of cold brand dyes i.e. in between 60-71°C temperatures. For example, Remazol, Levafix are medium brand dyes.
Hot Brand Reactive Dyes
This type of dye contains reactive groups of least reactivity. So high temperature is required for dyeing i.e. 72-93°C temperature is required for dyeing. For example, PROCION H, CIBACRON are hot brand dyes.
Recent Classification of Reactive Dyes
| Type | Reactivity | Condition | Temperature | Reactive Group |
|---|---|---|---|---|
|
Salt Controllable |
Low reactivity in alkaline conditions |
Appreciable substantivity, careful addition of salt is required. |
80°C temperature. |
Monochloro Triazine, Monofloro Triazine, Trichloro Pyrimidine. |
|
Alkali controllable |
High reactivity |
Moderate substantivity, careful control of the addition of alkali is required. |
Applicable at low temperature. |
Dichloro Triazine, Diflorochloro Pyrimidine, Vinyl Sulphone. |
|
Temperature controllable |
– |
Can be applied in neutral conditions |
Applicable in high temperatures. |
NT. |
Dyeing Mechanism of Reactive Dyes
The dyeing of material with reactive dye takes place in 3 stages –

- Exhaustion of dye in presence of electrolyte or dye absorption
- Fixation under the influence of alkali
- Wash-off of the unfixed dye from material surface
Absorption of Reactive Dyes
When fibre is immersed in dye liquor, an electrolyte is added to assist the exhaustion of dye. Here NaCl is used as the electrolyte. This electrolyte neutralizes cotton and helps absorption. So when the textile material is introduces to dye liquor the dye is exhausted on to the fibre.
Fixation of Reactive Dyes
Fixation of dye means the reaction of reactive group of dye with terminal –OH or –NH2 group of fibre and thus forming strong covalent bond with the fibre .
This is an important phase, which is controlled by maintaining proper pH by adding alkali.The alkali used for creates proper pH in dye bath and work as the dye-fixing agent. The reactions that take place in this stage are shown below:
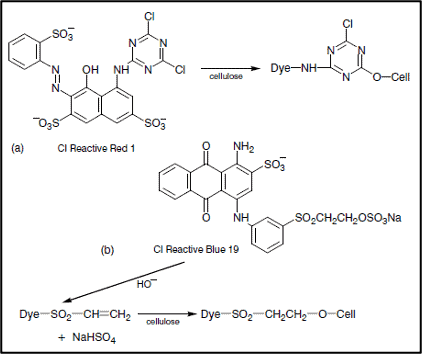
Types of Fixation Reaction
There are mainly two types of reactions that occur. These are –
Neucleofilic Substitution Reaction
Cell–OH + HO– ⇒ Cell–O– + H2O
Cell–O–+ Dye–Cl ⇒ Cell–O–Dye + Cl–
Neucleofilic Addition Reaction
Cell–O–+ Dye–SO2–CH=CH2 ⇒ Dye–SO2–CH=CH2–O–Cell
Wash-off of Reactive Dyes
As the dyeing is completed, a good wash must be applied to the material to remove extra and unfixed dyes from material surface. This is necessary for level dyeing and good wash-fastness. It is done by a series of hot wash, cold wash and soap solution wash.
Properties of Reactive Dyes
- All types of shades are available from these dyes.
- Reactive dyes are found in powder, liquid, and print-paste form.
- Reactive dyes are soluble in water.
- They have very good light fastness with a rating of 6. The dyes have very stable electron arrangements and can protect the degrading effect of ultra-violet rays.
- Textile materials dyed with reactive dyes have very good wash fastness with a rating of 4-5 due to strong covalent bonds formed between fibre polymer and reactive group of dye.
- Reactive dye gives brighter shades and has moderate rubbing fastness.
- Reactive dyes have good perspiration fastness with rating 4-5.
Factors to Be Considered During Reactive Dyeing
- pH – Strong alkaline pH is required ranging from 10.5-11.2.
- Temperature – Dyeing temperature depends on the brand of dye used.
- Concentration of electrolyte – The depth of shade is the determining factor of electrolyte concentration.
- Time – Ranges from 60-90 minutes.
- Liquor ratio – Higher liquor ratio gives better efficiency.
Reactive Dye Hydrolysis
Reactive dyes are activated when used to color cellulose in an alkaline solution. However, if the dye’s concentration is kept for a long period of time, it will eventually lose its potency. The dye then reacts with the hydroxyl group of water. This process in which a dye interacts with water is known as reactive dye hydrolysis.
The decomposition of the dye proceeds in two stages. The concentration of dye increases at first, followed by a decrease. The concentration of hydroxyl compound rises continuously as a result of this hydrolysis process. Then the hydroxyl compound is unable to react with the dye
Stripping of Reactive Dyes
The reactive dye cannot be satisfactorily stripped from fibre due to the covalent bond between dye molecule and fibre. Stripping becomes necessary when uneven dyeing occurs.
Partial Stripping of Reactive Dyes
Partial stripping is obtained by treating the dyed fabric with dilute acetic acid or formic acid. Here the temperature is raised to 70-100°C. The amount of acid used is as below:
- Glacial acetic acid: 5-10 parts
- With water: 1000 parts
Or
- Formic acid: 2.5 to 10 parts
- With water: 1000 parts
- Temperature: 70 – 100°C
- Time: until desired shade is obtained.
Advantages of Reactive Dyeing
- Brilliant, bright colours
- Permanency of the colour
- Covalent fixation – high Wash Fastness (WF)
- Various temperatures, including low energy (cold dyeing)
Disadvantages of Reactive Dyeing
- Incomplete fixation (problem with hydrolysis)
- Need for wash-off (for high WF)
- Need for high concentrations of salt
- Color is not easily removed by effluent treatment processes and in many cases, the dyes are not readily biodegradable
Precautions for Reactive Dyeing
- Hot water should not be used for high reactive dyes because there will be a possibility of hydrolysis.
- Prepared dye solution can not be stored for later use also because of dye hydrolysis.
- For making print paste, low reactive dyes are used.
- It is dangerous if there is the inhalation of the dust of reactive dyes, so a protective mask is required.
- A limited storage period is applicable to most of the reactive dye.
How Does Temperature Affect the Dyeing Process with Fiber Reactive Dyes?
Temperature’s impact on fiber reactive dyes is significant in the dyeing process. It mainly depends on the type of reactive dye you are using.
Generally speaking, higher temperatures help facilitate the chemical reaction between the dye and the fibers, resulting in a faster and more uniform color absorption. At the same time, it also promotes a higher rate of hydrolysis.
Frequently Asked Questions
1. Why are bifunctional reactive dyes preferable?
They have more than one identical functional group present in their structure. So, the degree of fixation is higher because if one functional group gets hydrolyzed, others will remain unhydrolyzed. Also, strict process control is not necessary.
2. Why reactive dyes were developed?
The main reason behind the discovery of reactive dye is that direct dyes have inferior wash fastness properties with cotton. Also, the dyeing of cotton with sulfur and vat dyes includes a very complicated process.
3. When did we discover the fiber reactive dyes?
During the 1900s, the idea of incorporating dyes with a covalent bond into the fiber was first generated. In 1955, Stephen and Rattee (ICI, England) first dyed cotton with dichlorotriazine reactive group-containing dye.
4. What is dye hydrolysis in reactive dyeing?
The most common problem of reactive dyeing is dye hydrolysis. In alkaline conditions, hydroxide ion also reacts with the reactive group of the dye like the fiber, and hydrolyzed dye is generated. The hydrolyzed dye can not react with fiber, and the efficiency of the fixation is decreased.
After dyeing, the hydrolyzed dye should be removed by washing. Otherwise, the wash fastness will be reduced.
5. Are reactive dyes colorfast?
They show good to excellent fastness to different wet processing, although incomplete removal of unreacted and hydrolyzed dye causes poor washing fastness. Lightfastness is moderate to good. Fastness to peroxides and bleaching by chlorine is also moderate.
6. Can I use a fiber reactive dye for tie dye?
Of course, you can tie dye with reactive dyes. In fact, this is the type of dye molecules that are marketed as Procion dyes for cellulosic fibers. Their use is not limited to solid colors only.
REFERENCE
- Textile Preparation and Dyeing by A K Roy
- Chakraborty, J. N. (2015). Fundamentals and practices in colouration of textiles. WPI Publishing.
- Shenai, V. A. (1983). Chemistry of dyes and principles of dyeing.
- Mahapatra, N. N. (2016). Textile dyes.
- Shore, J., & Society of Dyers and Colourists (Eds.). (2002). Colorants and auxiliaries: organic chemistry and application properteis. Vol. 1: Colorants (2. ed.). Bradford, West Yorkshire: Society of Dyers and Colourists.
- Shore, J., & Society of Dyers and Colourists (Eds.). (1995). Cellulosics dyeing. Bradford, West Yorkshire, England: Society of Dyers and Colourists.
- Broadbent, A. D. (2001). Basic principles of textile coloration. Bradford, West Yorkshire: Society of Dyers and Colourists.

